The pharmaceuticals and healthcare sector are the most significant area, where rules are crucial to follow. To conduct a stock audit one can effectively manage their inventories as it plays a crucial role in upholding quality, integrity, and patient well-being. It includes the regulation and complexity in the supply network, and the importance of stock audit services in the pharmaceutical and healthcare sector is significant for successful management in the operation of pharmaceuticals and healthcare. Join the Bizfoc to explore the details of stock audits in this crucial industry, learning about their importance, challenges, and best practices for ensuring optimal performance and regulatory compliance.
A stock audit is also known for the name of inventory audit, which ensures the reliability of medicines, medical tools, and medications. It includes the physical counting inventory and comparing it with the records already mentioned. The stock audit of pharmaceuticals and healthcare helps businesses to recognize inconsistencies such as theft, drugs, and missing supplies. The regular audits are necessary for the protection of patient, financial management and make sure the facilities of the resources needed to smoothly run the operation.
The healthcare and pharmaceutical companies need to follow the guidelines specified by the regulatory bodies such as Food and Drug Administration (FDA), and local health agencies. The regulations help in the reliability of stock management and regular audits to make sure the protection with the relevant medicinal supplies and medications. There are different stock audits rules, which depends on country to country. However, it includes the reliability of records, ensuring the relevant storage conditions, and recognizing counterfeit or expired medicines. If the pharmaceutical and healthcare companies are failed to comply with these rules will leads to severe consequences like risking the company's goodwill and reputation, recalls of items, and financial penalties. It is essential for the companies to keep informed as per the regulatory requirements.
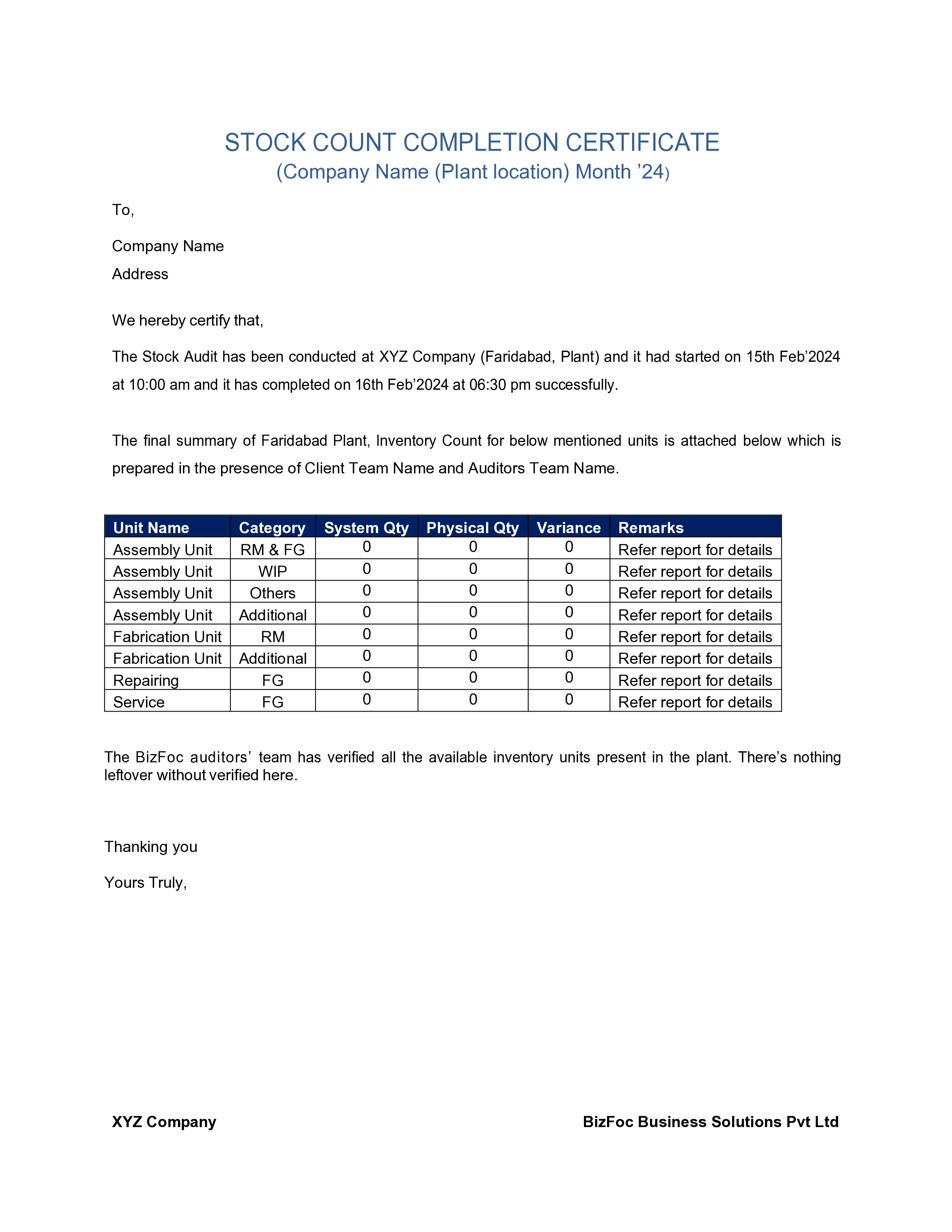
The inventory checklist generally includes the following details:
Mention the purpose and scope of the stock audit, which involves particular areas like expiration dates, inventory accuracy, and storage conditions. Collect appropriate documents like purchase orders, inventory records, and invoices. If the electronic inventory management system is available, then use it for gathering data.
It has to be kept in mind that compliance must be done as per regulatory standards by doing a physical examination and verifying numbers, product integrity, and expiration dates.
Inventory records need to verify for completeness, and accuracy, comparing them to physical inventory counts. Recognize any irregularities or discrepancies for further investigation.
Evaluate inventory practices to comply with regulatory requirements like Good Distribution Practices (GDP) and Good Manufacturing Practices (GMP). Recognize risks about the counterfeit or expired medications, inventory inaccuracies, and inappropriate storage. Make strategies to resolve risks and prevent their recurrence.
Need to make report for the stock audit findings that involves concern areas or discrepancies, and recommend suggestions for corrective action. Recognize relevant shareholders and top management to discuss these incurrences, and recommending to implement the outcomes.
Make sure to comply constantly and need continuous improvement in inventory management practices by monitoring the execution of corrective actions and taking follow-up audits as required.
There is various factors in which fess for stock audit in pharmaceuticals and healthcare generally depends on the complexity of inventory management systems, scope of the audit, and scale of the organization. Typically, based on the volume of inventory, specialized expertise required, number of locations, and regulatory compliance needs. Fees can be charged from hourly consultation and evaluation or fixed rate per audit.
Bizfoc has industry specific knowledge, manages risks, specializes in techniques and tools, regulatory compliances, and strategic insights. It is here to offer you a hassle-free services like stock audit services in pharmaceuticals and healthcare industry. Opting for Bizfoc as your choice for inventory/stock auditing is a vital decision that reflects a dedication to accuracy, transparency, and operational excellence.
In the dynamic realm of healthcare and pharmaceuticals, the significance of stock audit services cannot be overstated. Our exploration concludes with the realization that partnering with a reputable stock audit firm is not just a choice but a strategic imperative. These stock audit firms serve as trusted allies, guiding organizations through the intricacies of inventory management with precision and expertise.
Their insights can assist businesses in navigating challenges, optimizing processes, and ultimately upholding their commitment to delivering quality care and innovation to communities worldwide. Stock audit firms stand out as a sign of reliability and trust in an ever-changing landscape in the journey towards excellence.
To avail our stock audit service for healthcare and pharmaceutical industry, just get in touch with our experienced stock audit team and discuss the next steps.
To conduct a stock audit in pharmaceuticals and healthcare, review inventories, check expiry dates, reconcile discrepancies, assess storage conditions, ensure compliance with regulations, and execute management to prevent losses and ensure efficiency.
The objective of stock audit in pharmaceuticals and healthcare industry is to comply with regulatory regulations, prevent stockouts, protect against counterfeit or expired items, reduce discrepancies, and optimize inventory levels.
The process involves monitoring inventory levels, ordering medications as needed, tracking expiration dates, conducting regular audits, and adjusting stock levels based on demand and usage patterns.
Pharma companies should conduct stock audits regularly, ideally on a quarterly basis, to maintain accurate inventory records, ensure compliance with regulations, identify discrepancies promptly, and mitigate operational risks effectively.